Abstract
Introduction: Minimal residual disease (MRD) is an emerging clinical endpoint in chronic lymphocytic leukemia (CLL). Multiple studies have demonstrated that achieving MRD below 1 in 10,000 (10-4) CLL cells per leukocyte in the blood and/or bone marrow corresponds to longer progression-free survival (PFS) (Owen et al. Leuk Lymphoma . 2017). With the introduction of venetoclax in combination with rituximab high rates (>50%) of MRD negativity (<10-4) may be achieved in relapsed or refractory (R/R) CLL. However, the duration to treat patients to achieve this low level of MRD, and the duration patients should be treated with venetoclax once MRD negativity (<10-4) is achieved, has not been fully elucidated. Therefore, the objective of this research was to develop an integrated semi-mechanistic model of the kinetics of MRD in R/R CLL in response to venetoclax and rituximab therapy in order to evaluate such treatment questions.
Methods: Data from 4 phase I and II clinical studies of venetoclax monotherapy and rituximab (6-cycles) combination therapy in R/R CLL were combined for the analysis. An integrated semi-mechanistic pharmacokinetic-pharmacodynamic (PK-PD) MRD model was constructed that accounted for venetoclax PK, rituximab treatment, absolute lymphocyte count (ALC), and blood and BM MRD data. Upon validation of the MRD negativity (<10-4) rates in the blood and BM, the integrated model was used to evaluate the time course of achieving MRD negativity at the 10-4 threshold of venetoclax combined with 6 cycles of rituximab treatment. In addition, the MRD negativity (<10-4) rates were compared between those achieved with continuous venetoclax treatment until progression and those achieved with a defined 24 months of venetoclax treatment.
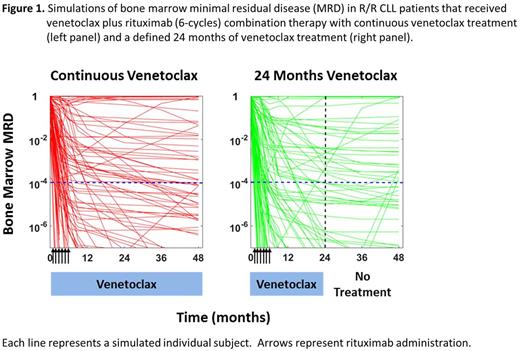
Results: A total of 402 R/R CLL patients, including 49 patients receiving venetoclax and rituximab combination therapy, were incorporated into the dataset. Model predictions of BM MRD negativity (<10-4) in patients administered venetoclax monotherapy and in combination therapy with rituximab were both in good agreement with the observed results. Simulations of venetoclax in combination with rituximab across all R/R CLL patients estimated rates of BM MRD below 10-4 of 48% (90% CI: [43 - 52%]), 60% (90% CI: [53 - 64%]), and 63% (90% CI: [57 - 67%]) at 6, 12, and 24 months of venetoclax treatment, respectively (Figure 1). Continued venetoclax treatment beyond 12 months was predicted to increase the MRD response, even when MRD negativity at 10-4 was reached within 1 year of treatment, with an improved depth of the MRD achieved. Consistent with the observed data, a diversity of responses to treatment were captured in the simulations, with many individual patients predicted to rapidly respond, some predicted to achieve deep responses and then relapse, and a minority of patients predicted to have little-to-no response to the combination therapy. Continued venetoclax treatment beyond 24 months was predicted to result in minimal additional benefit, with comparable rates of MRD below 10-4 to that achieved with stopping therapy. The simulation-based estimated rate of MRD negativity (<10-4) at 48 months with continuous venetoclax treatment was 66% (95% CI: [63 - 70%]) (Figure 1, left panel) compared to an estimated rate of MRD negativity (<10-4) of 64% (95% CI: [61 - 70%]) at 48 months with venetoclax treatment stopping at 24 months (Figure 1, right panel). As additional MRD assessments become available from patients off of venetoclax treatment, these estimates of MRD durability after stopping all therapy may be further refined.
Conclusions: The integrated semi-mechanistic model of MRD in R/R CLL patients was able to describe the kinetics of MRD in response to venetoclax monotherapy and combination therapy with rituximab. Simulations indicated that treatment of patients with venetoclax in combination with rituximab beyond 2 years is unlikely to achieve further reductions in the rate of MRD negativity (<10-4), even years after stopping venetoclax.
Gopalakrishnan: AbbVie: Employment, Equity Ownership. Chyla: AbbVie: Employment, Equity Ownership. Mensing: AbbVie: Employment, Equity Ownership, Patents & Royalties. Menon: AbbVie: Employment, Equity Ownership. Humerickhouse: AbbVie: Employment, Equity Ownership. Awni: AbbVie: Employment, Equity Ownership. Salem: AbbVie: Employment, Equity Ownership. Freise: AbbVie: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal